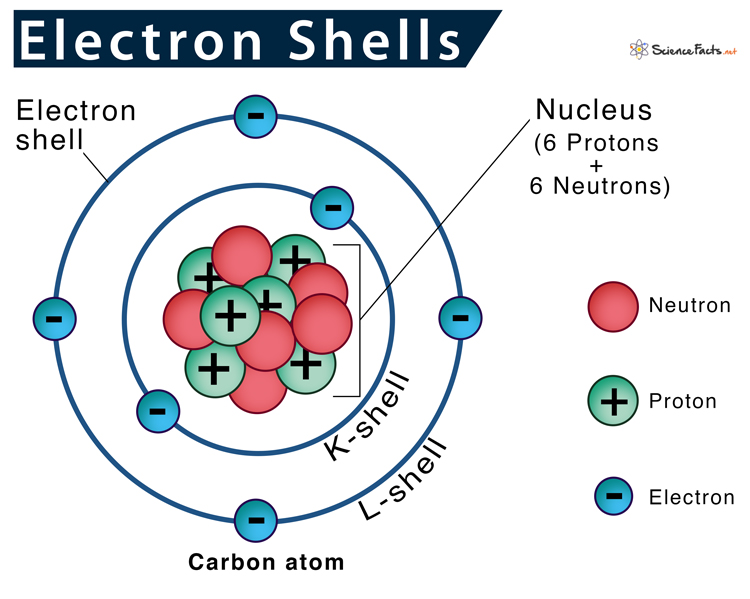
Iron Electron Shell Diagram . iron ion(fe 2+, fe 3+) electron configuration. iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of. The ground state electron configuration of iron is 1s 2 2s 2 2p 6 3s 2. the electron configurations and orbital diagrams of these four elements are: how to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the. electronic configuration of iron is [ar] 3d 6 4s 2. here are electron shell atom diagrams for the elements, ordered by increasing atomic number. shells, subshells, and orbitals. the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and. Irons peculiar crystalline structure and electronic configuration make them.
from www.animalia-life.club
The ground state electron configuration of iron is 1s 2 2s 2 2p 6 3s 2. the electron configurations and orbital diagrams of these four elements are: how to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the. the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and. shells, subshells, and orbitals. iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of. iron ion(fe 2+, fe 3+) electron configuration. electronic configuration of iron is [ar] 3d 6 4s 2. here are electron shell atom diagrams for the elements, ordered by increasing atomic number. Irons peculiar crystalline structure and electronic configuration make them.
Electron Shell Diagram
Iron Electron Shell Diagram shells, subshells, and orbitals. iron ion(fe 2+, fe 3+) electron configuration. how to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the. electronic configuration of iron is [ar] 3d 6 4s 2. the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and. Irons peculiar crystalline structure and electronic configuration make them. here are electron shell atom diagrams for the elements, ordered by increasing atomic number. shells, subshells, and orbitals. The ground state electron configuration of iron is 1s 2 2s 2 2p 6 3s 2. iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of. the electron configurations and orbital diagrams of these four elements are:
From www.sciencephoto.com
Iron, atomic structure Stock Image C018/3707 Science Photo Library Iron Electron Shell Diagram iron ion(fe 2+, fe 3+) electron configuration. here are electron shell atom diagrams for the elements, ordered by increasing atomic number. Irons peculiar crystalline structure and electronic configuration make them. how to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the. shells, subshells, and. Iron Electron Shell Diagram.
From www.shutterstock.com
This Diagram Shows Electron Shell Configuration Stock Vector 329198879 Iron Electron Shell Diagram iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of. Irons peculiar crystalline structure and electronic configuration make them. how to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the. shells, subshells, and. Iron Electron Shell Diagram.
From www.webelements.com
Elements Periodic Table » Iron » properties of free atoms Iron Electron Shell Diagram the electron configurations and orbital diagrams of these four elements are: here are electron shell atom diagrams for the elements, ordered by increasing atomic number. electronic configuration of iron is [ar] 3d 6 4s 2. how to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to. Iron Electron Shell Diagram.
From www.bigstockphoto.com
Iron. Atom Structure Vector & Photo Bigstock Iron Electron Shell Diagram the electron configurations and orbital diagrams of these four elements are: iron ion(fe 2+, fe 3+) electron configuration. here are electron shell atom diagrams for the elements, ordered by increasing atomic number. how to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the. . Iron Electron Shell Diagram.
From www.pngjoy.com
Image Example Electron Shell Diagram Of Iron 1521x1551 (23855848 Iron Electron Shell Diagram the electron configurations and orbital diagrams of these four elements are: how to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the. here are electron shell atom diagrams for the elements, ordered by increasing atomic number. The ground state electron configuration of iron is 1s. Iron Electron Shell Diagram.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Iron Electron Shell Diagram The ground state electron configuration of iron is 1s 2 2s 2 2p 6 3s 2. how to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the. iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an. Iron Electron Shell Diagram.
From valenceelectrons.com
How to Find the Valence Electrons for Iron (Fe)? Iron Electron Shell Diagram how to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the. The ground state electron configuration of iron is 1s 2 2s 2 2p 6 3s 2. the electron configurations and orbital diagrams of these four elements are: electronic configuration of iron is [ar] 3d. Iron Electron Shell Diagram.
From www.pinterest.com
FileElectron shell 021 Scandium.svg Wikimedia Commons Hierro Iron Electron Shell Diagram shells, subshells, and orbitals. iron ion(fe 2+, fe 3+) electron configuration. electronic configuration of iron is [ar] 3d 6 4s 2. iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of. the electron configurations and orbital diagrams of these four elements are: . Iron Electron Shell Diagram.
From www.istockphoto.com
Te Tellurium Element Information Facts Properties Trends Uses And Iron Electron Shell Diagram Irons peculiar crystalline structure and electronic configuration make them. electronic configuration of iron is [ar] 3d 6 4s 2. how to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the. the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17. Iron Electron Shell Diagram.
From www.alamy.com
Symbol and electron diagram for Iron illustration Stock Vector Image Iron Electron Shell Diagram the electron configurations and orbital diagrams of these four elements are: how to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the. iron ion(fe 2+, fe 3+) electron configuration. the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17. Iron Electron Shell Diagram.
From enginedbtersanctus.z22.web.core.windows.net
Iron Electron Config With Orbital Diagram Iron Electron Shell Diagram the electron configurations and orbital diagrams of these four elements are: shells, subshells, and orbitals. the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and. electronic configuration of iron is [ar] 3d 6 4s 2. here are electron shell atom diagrams for the elements, ordered by increasing atomic. Iron Electron Shell Diagram.
From pnghut.com
Electron Configuration Atomic Orbital Shell Energy Level Iron Iron Electron Shell Diagram how to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the. iron ion(fe 2+, fe 3+) electron configuration. the electron configurations and orbital diagrams of these four elements are: iron is a chemical element of the periodic table with chemical symbol fe and atomic. Iron Electron Shell Diagram.
From stock.adobe.com
illustration of chemistry, Electron shell diagram,atomic shell is a Iron Electron Shell Diagram electronic configuration of iron is [ar] 3d 6 4s 2. iron ion(fe 2+, fe 3+) electron configuration. the electron configurations and orbital diagrams of these four elements are: shells, subshells, and orbitals. how to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the.. Iron Electron Shell Diagram.
From www.schoolmykids.com
Iron (Fe) Element Information, Facts, Properties, Uses Periodic Iron Electron Shell Diagram the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and. iron ion(fe 2+, fe 3+) electron configuration. the electron configurations and orbital diagrams of these four elements are: iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight. Iron Electron Shell Diagram.
From www.worldanvil.com
Iron Material in The Spaceport World Anvil Iron Electron Shell Diagram how to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the. iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of. Irons peculiar crystalline structure and electronic configuration make them. shells, subshells, and. Iron Electron Shell Diagram.
From cathy.devdungeon.com
Draw The Electron Configuration For A Neutral Atom Of Iron. Iron Electron Shell Diagram iron is a chemical element of the periodic table with chemical symbol fe and atomic number 26 with an atomic weight of. the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and. here are electron shell atom diagrams for the elements, ordered by increasing atomic number. iron ion(fe 2+,. Iron Electron Shell Diagram.
From dxoiremot.blob.core.windows.net
Element Iron Works at Robert Davis blog Iron Electron Shell Diagram how to write the electron configuration for iron (fe) in order to write the iron electron configuration we first need to know the. Irons peculiar crystalline structure and electronic configuration make them. electronic configuration of iron is [ar] 3d 6 4s 2. here are electron shell atom diagrams for the elements, ordered by increasing atomic number. . Iron Electron Shell Diagram.
From autoctrls.com
The Fascinating Structure of Electron Shells A Diagrammatic Exploration Iron Electron Shell Diagram the electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and. shells, subshells, and orbitals. iron ion(fe 2+, fe 3+) electron configuration. electronic configuration of iron is [ar] 3d 6 4s 2. The ground state electron configuration of iron is 1s 2 2s 2 2p 6 3s 2. the. Iron Electron Shell Diagram.